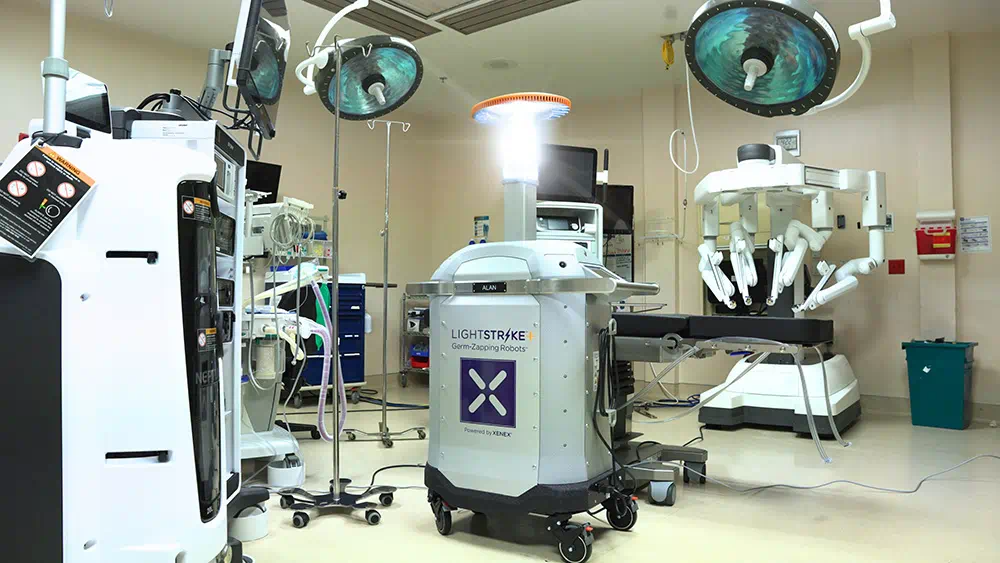
Hogan Lovells advises Xenex in securing FDA De Novo authorization for its UV microbial reduction robot for use in health care facilities
Washington, D.C., 13 September 2023 — Global law firm Hogan Lovells advised Xenex Disinfection Services, Inc. (Xenex), a leading company specializing in ultraviolet (UV) robots used to destroy pathogens in health care facilities, in securing FDA market authorization for its LightStrike+ medical device. LightStrike+ performs microbial reduction using xenon lamp technology that emits high-intensity, broad-spectrum UV light to quickly reduce the number of pathogens on surfaces. The device is intended for use in unoccupied operating rooms, hospital rooms, and other clinical settings where non-critical medical devices are present, as an adjunct to existing manual cleaning and disinfection practices. More about the approval, which was granted 1 September, 2023, can be found here.
The authorization creates an entirely new product classification identified as “whole room microbial reduction devices” – strengthening Xenex‘s leading position by obtaining FDA authorization to market LightStrike+ as a medical device. The FDA’s authorization was granted following substantial testing and data collection, and by leveraging Xenex’s robust scientific evidence published in peer-reviewed articles about the device’s safety and effectiveness.
LightStrike+ is the only whole room microbial reduction device authorized to be marketed to U.S. health care facilities.
Hogan Lovells Medical Device & Technology partner Jodi Scott, based in Denver, said: "We are proud to have worked closely with FDA together with our client, whose unique UV microbial reduction device will not only help health care professionals make thoughtful, data-driven decisions, but also improve patient safety, a top priority for Xenex. We look forward to continue working with Xenex on similar innovative technologies moving forward.”
In addition to Scott, the Hogan Lovells team included Medical Device & Technology Practice Area Lead Randy Prebula, counsel Suzanne Levy Friedman, associate Sanchita Bose, and Director of Regulatory Sciences Alex Smith (all Washington, D.C.).